The surfaces of most traditional biochips have a water contact angle greater than 30 degrees. These surfaces contain hydrophobic domains in nanoscale, which can have hydrophobic interaction with the hydrophobic domains of proteins and cause none-specific adsorption.
This adsorption is unstable and generates irreproducible signals. Hydrophobic interaction can also cause proteins to change conformation and to lose activity. Therefore, biocompatible surfaces are usually hydrophilic, ideally, with water contact angle less than 30 degrees. In addition, the surfaces of biochips need to have an abundance
of active chemical groups (i.e., carboxyl, amine, or thiol) to covalently bind to biomolecules. These groups are all polar and lead to the hydrophilicity of a surface. Sofchip supplies a
large variety of hydrophilic polymer coated biochips. They have the following features:
- Biocompatible. The coatings of the biochips are made from well selected hydrophilic polymers. They present a water-like environment to keep the native conformation of biomolecules and retain their bioactivities.
- Low none-specific adsorption. The hydrophilic nature of these biochips eliminates the hydrophobic interaction that is the main driving force of none-specific adsorption. No blocking agents are required .
- Ultra smooth surface with various thickness. The roughness of the surfaces is within a few nanometers that gives a very low coefficient of variation. The thickness of the coatings ranges from two to a hundred nanometers, satisfying different applications.
- High stability. Coated surfaces can retain their functionalities for up to years. They can withstand sufficient washing with most general solvents/detergents.
- All biochips are manufactured under strict quality control and in an ultraclean environment. They will give you highly reproducible experimental data.
For efficient immobilization, the surface polymer should be compatible (not just biocompatible) with the target biomolecule, which means they can access each other. The compatibility is affected by the charge, polarity, and structure of both the surface polymer and the biomolecules.
Due to the large diversity of biomolecules, there is no universal biochip suitable for so many biomolecules. Sofchip provides various types of polymer coatings to be compatible with different biomolecules. The coatings are classfied into three catagories: monolayers, polysaccharides,
and synthetic polymers. The monolayer based coatings have shortest chains. A self-assembly monolayer (SAM) based SPR chip made with traditional procedures degrades quickly in air. The traditional silane based microarray sides have contact angles ranging from 30 to 150 degrees.
Sofchip uses an improved procedure to manufacture better performing monolayer based biochips. The polysaccharide based coatings are very stable. Polysaccharides (e.g., dextran and alginate acid) have long and bulky chains bearing hydroxyl groups. The chains extend out from the surface allowing biomolecules easy access. The hydroxyl groups form hydrogen
bonds with water molecules and resist none-specific adsorption. Synthetic polymers have long, slim and straight chains and form a brush-like structure on a surface. In addition to the advantages of polysaccharides, the slim chains leave more space for biomolecules to diffuse freely in and bind to the polymer matrix.
Frequently asked Questions
Will a third party SPR sensor chip cause problems with my SPR instrument?
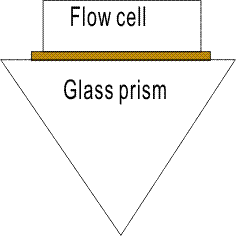
The only parts in SPR instruments that interface a sensor chip are the plastic flow cell and the glass prism (see figure below) both of which are robust. The sensor chip will not touch any sensitive parts in SPR instruments.
So the sensor chip will not cause problems with SPR instruments.
How do I know a third party SPR sensor chip is compatible with my SPR instrument?
A simple way to verify the compatibility is to test with water solution of sodium chloride after installing the chip. Inject various concentrations of sodium chloride solution (e.g., 10mg/ml, 20mg/ml, 30mg/ml, etc.) into the flow cell. Since the SPR signal response is proportional to the concentration,
you should see the signal goes up stepwise. It means the SPR signal responds correctly. You can also use this method to calibrate your system. SPR technique essentially measures the change of refractive index near the gold surface. It does not matter if the change is caused by the concentration change of sodium chloride or biomolecules.
Our SPR chips have been tested on all major vendors' SPR instruments, and we did not see any compatibility problem.
How to replace the gold chip from a plastic holder?
- Remove the used gold chip from the plastic substrate with a blade. The original glue is soft and can be easily removed.
- Distribute the glue evenly on a sheet to form a thin layer (0.2-0.4 mm thick).
- Stamp the glue to the edges of the window of the plastic substrate with our product(catalog number 9800101-3 or 9800102-3 ).
- Mount the new gold plate on the substrate. Avoid any contamination of the gold chip.
- Clean out any glue residue on the plastic substrate with lab tissue wetted with ethanol.
Practice with used chips several times before mounting the new chip. Most glues (e.g., super glue, epoxy glue, silicone glue, etc.) will work. The chip replacement procedure normally takes less than ten minutes.
 |  |  |
| Plastic holder with chip removed, | Plastic holder aligned with glue stamp | Plastic holder with chip mounted |
How to identify the gold surface and the glass surface of a SPR chip?
The glass surface should face towards prism and the gold surface should face towards flow cell when you install a SPR chip. There are three ways to identify these two surfaces.
The first way is by color. the glass surface is a little more brownish compared to yellowish gold surface. This slight difference can be seen in certain direction of room light.
The second way is to look through a edge from the opposite direction. You are facing the glass surface if you see a transparent edge. The third method is to slightly scratch the surface at a corner with a sharp tool. You will see marks left on gold surface, but not on glass surface.
What are the differences among the three synthetic polymers in SPR chips and microarray slides?
The three polymers are all hydrophilic polymers with good biocompatibility. They differ in charge, polarity, and structure. It is often found that the chips carrying the same functional group but different underline polymer matrixes can have very different responses
to immobilizing different biomolecules. These three polymers are selected to match different biomolecules complementary to polysaccharide based coatings.
How to select a proper sensor chip?
Several factors should be considered to select a proper biochip. First the biochip should bear the desired functional groups that can react with the target biomolecule. Second the surface polymer should be compatible with the biomolecule. Predicting the compatibility
is not easy. In many cases biomolecules and the surface polymers can access each other if they have opposite signs of charge (the charges of a protein can be altered by adjusting the pH of the buffer according to its isoelectric point). Third, a thick coating provides
more immobilization sites if the biomolecules can diffuse into the surface matrix. Custom coating services are also available if you cannot find the desired chip in our product listing.
How stable is the surface coating from Sofchip?
All coatings from Sofchip are bound to surfaces through high density of covalent bonds. Most of these coatings can withstand hard wash (e.g., ultrasonic bath) with most lab solvents and can be stored for long time. But for best performance, we don’t recommend treating the
chips at extreme condition.
How to increase the immobilization capacity?
The signal responses from hydrophilic surfaces during biomolecular immobilization may sometimes not seem strong since the none-specific adsorption is greatly reduced, but these signals are stable and reproducible. If these signals are too weak, try:
- Adjusting the pH of the immobilization buffer, usually one lower than the isoelectric point (pI) of the protein if using carboxyl functionalized chip.
- Lowering the salt concentration of the buffer.
- Changing to another polymer coating for better compatibility.
- If no hydrophilic chips work, it means there are not enough reactive groups exposed outside of the biomolecules. Try hydrophobic chips that utilize hydrophobic interaction or UV-initiator modified chips that do not rely on any functional groups.
How to activate carboxylate functionalized chips?
- Prepare 80 mg EDC (catalog number 152432) and 12 mg NHS (catalog number 7161131) in 2 ml deionized water.
- Soak the chip in above solution for twenty minutes.
- Wash the chip with deionized water three times and dry it with nitrogen gas.
For best performance, use the activated chip as soon as possible.
How to control the spreading of the spots from a microarrayer?
The hydrophilicity of carboxyl functionalized chips will be reduced after EDC/NHS activation, and thus the spreading of the spots from a microarrayer can be reduced. Custom coating for desired hydrophilicity from Sofchip is also available.
How to test the reliability of your SPR sensor?
Before starting any complex experiment, we recommend you test the reliability of your SPR instrument using a simple method. Select a water dissolvable thiol compound (e.g., catalog number 7125112) and study its binding to a plain gold surface. You should see a SPR response
above 7000 RU against water. This signal is very stable and cannot be washed out with any lab solvents. If your system fails to give such a response, there is a problem with your SPR sensor or your operating procedure.
What are the features of the conjugated gold nanoparticles from Sofchip?
The features are more stable, higher density of functional groups, and unique underline polymer matrix for good biocompatibility, reduced none-specific adsorption and easy access of functional groups.